| Livestock Research for Rural Development 37 (2) 2025 | LRRD Search | LRRD Misssion | Guide for preparation of papers | LRRD Newsletter | Citation of this paper |
The experiment aimed to determine the effect of replacing fishmeal protein with larval meal protein on growth performance, meat chemical composition, digestive enzyme activity and main haematological indicators of seabass fingerlings. Total 225 seabass fingerlings of 14.4 g were randomly located to 5 dietary treatments and 3 replicates, namely AT0, AT25, AT50, AT75 and AT100, in which, 0%, 25%, 50%, 75% and 100% fishmeal protein replaced by black soldier fly larvae meal. The results showed that increasing the protein replacement levels at up to 50% without effect on growth rate and yield and the chemical composition of meat and some digestive enzymes as well as haematological indices of seabass fingerlings. In recommendation, replacement up to 50% of fishmeal protein (or 214 g/kg diet) by black soldier fly larvae meal can be applied in diets for seabass fingerlings rearing in freshwater.
Keyword: creatinine, hepatosomatic index, lipase, protease, urea
Asian seabass ( Lates calcarifer Bloch, 1790) is a catadromous species (fish that live most of their life in freshwater, but spawn in saltwater) (Vij et al 2014) and widely distributed in tropical and subtropical regions of Asia - Pacific. This species has also been utilized in aquaculture for the past several decades (Pethiyagoda and Gill 2012; Hassan et al 2021) in many countries such as Indonesia, Malaysia, China, Australia, Thailand, etc. (Schipp 2007; Weerakit 2017; Irmawati et al 2020). In Viet Nam, this species has been cultivated in various regions, including Thua Thien Hue area in which the freshwater and brackish water aquacultures are located (Lan et al 2022). In practice, seabass was fed mainly with trash fish or industrial pellets with a high fishmeal proportion, which has increased feed costs, that usually accounted for 60-70% of production costs (Nhan et al 2022).
Many present studies indicated that black soldier fly ( Hermetia illucens Linnaeus, 1758) larvae (BSFL) protein is one of promising rich-protein feeds and can replace fully or partly fishmeal for aquaculture (Lan et al 2022; Nghia et al 2023; Suong et al 2023; Manh et al 2023; Mathai et al 2024; Kim et al 2024). Lan et al (2022) reported that partly replacement of trash fish by fresh larvae improved growth rate of seabass rearing in brackish water and freshwater but fully replacement impaired their growth performance. Similarly, Manh et al (2023) concluded that the inclusion of up to 12.9% BSFL meal (as diet dry matter) in diets didn’t affect growth performance and yield of snakehead fish. Suong et al (2023) found that growth performance of climbing perch has been improved when fishmeal protein replaced by BSFL protein up to 40% or 9.2% BSFL meal in diets. In addition, in the study on the effect of inclusion 5% of defatted and full fat BSFL meal in diets of red seabream, Kim et al (2024) observed no differences in weight gain, specific growth rate, feed conversion ratio, morphological parameters, plasma metabolites, plasma lysozyme, glutathione peroxidase and superoxide dismutase among the experimental groups. However, immunoglobulin M (IgM) in fish fed 5% defatted BSFL meal and 5% full fat BSFL meal were significantly higher than those in fish fed a control diet. Additionally, the fish in 5% defatted BSFL diet showed higher IgM levels than those in the other treatment groups.
Clearly, high proportion of BSFL meal in diets negatively affect growth rate of fish but the effect on composition of fish meat, digestive enzymes and blood indication is not yet known. The present study aimed therefore at determining the effects of using BSFL in the diet on growth, survival rate, chemical composition, digestive enzyme activity and some haematological indicators of seabass fingerlings.
Black soldier fly larvae (BSFL) were reared on a substrate of tofu by-product and harvested at the 7th day after culture under the temperature 26-30oC and humidity 70%. After harvesting, BSFL were washed with water several times to remove impurities, dried under 60oC and milled.
Seabass fingerlings were acclimatized for 7 days with experimental diets and simultaneously acclimatized to salinity from 10‰ to 0‰ before starting the experiment.
A total of 225 seabass fingerlings of 14.4 g live weight were randomly allocated to 5 diets and 3 replicates. The dietary treatments were namely AT0 - control diet based on fishmeal (FM) protein and AT25, AT50, AT75 and AT100 - replacing 25%, 50%, 75% and 100% of FM protein with black soldier fly larvae (BSFL) meal protein, respectively. The proportion of ingredients and nutritive value of all diets were presented in Table 1. Fish were kept with density of 75 fish/m3, fed on demand twice a day (8.00h and 17.00h) for 90 days. During the experiment, water was changed daily, each time about 30 - 50% of the water volume in the tank. Water quality parameters were checked periodically every 2 days.
Growth performance and yield, live weight (W), daily weight gain (DWG), specific growth rate (SGR), feed conversion ratio (FCR), survival rate (SR), yield (kg/m3), protein utilization efficiency (PER) and hepatosomatic index (HSI).
Blood plasma analysis, at the end of the experiment, fish blood were collected for analysis of Glucose (GLU), urea (URE), total protein (TP), creatinine (CRT), cholesterol (CHOL), triglycerides (TG), albumin (ALB), alanine transaminase (ALT), globulin (GLO) and aspartate aminotransferase (AST).
At the end of the experiment, fish’s stomach was collected to analyse digestive enzymes: pepsin enzyme by Kit - K446; trypsin enzyme by Kit - K771 of Biovison, US; protease enzyme by Cupp-Enyard (2008), lipase by Shirai and Jackson (1980); and amylase by Miller (1959).
Feed, larvae and fish meat samples were analysed for DM, N, EE and total ash according to AOAC (1990). Amino acids were analysed according to AOAC 994.12 (1997); and fatty acids were analysed according to ISO (2017).
|
Table 1. Ingredients and nutritive value of diets(1) |
|||||||
|
Item |
Treatments |
||||||
|
AT0 |
AT25 |
AT50 |
AT75 |
AT100 |
|||
|
Ingredients in diet (g/kg) |
|||||||
|
Fishmeal |
440 |
330 |
220 |
110 |
- |
||
|
Larvae meal |
- |
107 |
214 |
321 |
428 |
||
|
Soybean meal |
275 |
275 |
265 |
265 |
265 |
||
|
Maize meal |
100 |
106 |
87.0 |
78.9 |
70.5 |
||
|
Meizan wheat meal |
75 |
87 |
130 |
150.1 |
171.5 |
||
|
Simply soybean oil |
30 |
22.5 |
17 |
12.5 |
7.5 |
||
|
Moller’ Tran fish oil |
30 |
22.5 |
17 |
12.5 |
7.5 |
||
|
KC-POL(2) |
30 |
30 |
30 |
30 |
30 |
||
|
CMC(3) |
20 |
20 |
20 |
20 |
20 |
||
|
Total |
1.000 |
1.000 |
1.000 |
1.000 |
1.000 |
||
|
Nutritive value (% DM) |
|||||||
|
Dry matter (DM) |
94.9 |
94.9 |
95.1 |
95.2 |
95.7 |
||
|
Organic matter (OM) |
87.8 |
88.0 |
88.6 |
89.1 |
89.6 |
||
|
Crude protein (CP) |
44.2 |
43.3 |
43.4 |
43.3 |
42.8 |
||
|
Ether extract (EE) |
10.6 |
10.1 |
10.2 |
10.5 |
10.9 |
||
|
Crude fiber (CF) |
4.48 |
5.07 |
5.54 |
6.08 |
6.48 |
||
|
Total ash (TA) |
12.2 |
12.0 |
11.4 |
10.9 |
10.4 |
||
|
NFE(4) |
28.5 |
29.5 |
29.5 |
29.2 |
29.4 |
||
|
GE (MJ/kg)(5) |
20.3 |
20.2 |
20.3 |
20.5 |
20.7 |
||
|
Essential amino acid (% DM) |
|||||||
|
Arginine |
2.67 |
2.71 |
2.79 |
2.99 |
2.36 |
||
|
Histidine |
1.29 |
1.30 |
1.43 |
1.32 |
1.32 |
||
|
Isoleucine |
2.04 |
1.85 |
1.77 |
2.08 |
1.67 |
||
|
Leucine |
3.13 |
3.02 |
2.85 |
3.40 |
2.72 |
||
|
Lysine |
2.83 |
2.69 |
2.68 |
2.66 |
2.59 |
||
|
Methionine |
1.21 |
1.09 |
1.13 |
1.15 |
1.24 |
||
|
Phenylalanine |
1.04 |
2.01 |
2.17 |
2.46 |
2.01 |
||
|
Threonine |
1.82 |
1.92 |
1.98 |
2.10 |
1.79 |
||
|
Valine |
2.37 |
2.77 |
3.08 |
3.31 |
2.78 |
||
| (1)Analyzed (2)KC - POL contained in 1kg: Vitamin A (6.000.000 IU); D3 (1.000.000 IU); E (2.000 IU); K3 (1.000 mg); B1 (2.000 mg); B2 (3.000 mg); B6 (500 mg); B12 (1.000 mcg); Niacin Amide (6.000 mg); Na (2.520 mg); Ca-Pantothenate (5.000 mg); DL-Methionine (16.000 mg); Co (220 mg); Mn (140 mg); Fe (2.140 mg); K (3.740 mg); Zn (130 mg); L-Lysine (5.000 mg); Folic Acid (400 mg); (3)CMC: Carboxyl methyl cellulose; (4)NFE = 100 - (CP + EE + ash + CF); (5)Gross energy (MJ/kg) = 4.184 x [4143 + (56 x EE + 15 x CP - 44 x ash)]/1000 | |||||||
Experimental data are presented as mean value (M) and standard error of the mean (SEM). The data were statistically processed by ANOVA by the GLM application of Minitab 16.2 software (2010). The difference between the means is determined by the Tukey method with 95% confidence. Mathematical model for one factor design:
Y ij = µ + α i + eij(1)
In which, Y ij observed value; µ population effect; α i treatment effect; and e ij random error.
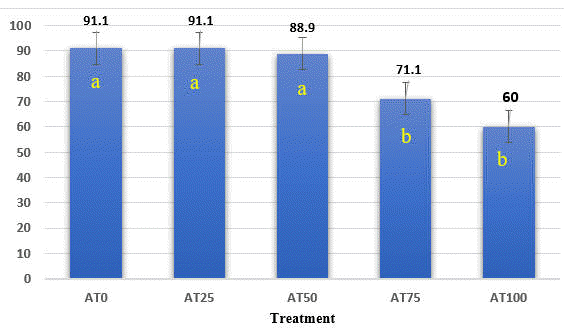
The survival rates ranged 60 - 91,1% (Figure 1.) and were lower in AT75 and AT100 than in other treatments (p<0.05). It means that replacing 75% to 100% FM protein by BSFL meal reduces significantly the survival rate of fish.
 |
| Figure 1. Survival rate of fish, (%) |
The survival rate in this study is lower than result of Katya et al (2018) and Magalhăes (2017), who reported that the survival rate of seabass in freshwater ranged 80.5 - 100%. In addition, Hoa and Dung (2016) reported that replacing 50% of FM protein by BSFL reduced the survival rate of snake head fish (Channa micropeltes), meanwhile, Thong and Lan (2019) found that replacing 75% FM by cricket meal didn’t affect survival rate of seabass (Psammoperca waigiensis).
In this study, the inclusion of BSFL meal in diets up to 42.8% as DM diet, which was higher than the level of <30% recommended by many authors. The results of this study showed that increasing the proportion of BSFL (more than 50% as FM protein or 21.4% as DM diet) reduced the survival rate of fish. On the other hand, the palatable diet and the balance of nutrients are very important because they are related to the ability to consume feed, digestibility of nutrients and the activity of fish digestive enzymes. The results of the survival rate in this study showed that adding BSFL to the diet at a level of 21.4% as DM (or 50% of fish meal protein) is suitable for raising seabass.
Results in Table 2 and Figure 2 show that increasing FM protein replacement levels declines fish growth performance (p<0.05) such as final weight, total and daily weight gain and specific growth rate and yield, particularly the levels above 50% as FM protein. There is no different growth performance of fish in the AT0 (FM diet) and AT25 (25% FM protein replacing by BSFL) and the fish yield in the AT0, AT25 and AT50 is higher than in AT75 and AT100 (p<0.05).
|
Table 2. Growth performance, protein efficiency, hepatosomatic index and yield of seabass fingerlings |
|||||||
|
Item |
Treatment |
p -value |
|||||
|
AT0 |
AT25 |
AT50 |
AT75 |
AT100 |
|||
|
Initial weight (g) |
14.4 |
14.5 |
14.5 |
14.4 |
14.4 |
0.866 |
|
|
Final weight (g) |
112.1a |
109.4a |
97.1b |
61.0c |
20.8d |
<.001 |
|
|
Total weight gain (g) |
97.7a |
94.9a |
82.6b |
46.5c |
6.42d |
<.001 |
|
|
Daily weight gain (g) |
1.09a |
1.06a |
0.92b |
0.52c |
0.07d |
<.001 |
|
|
SGR(%)# |
2.28a |
2.25a |
2.12b |
1.60c |
0.41d |
<.001 |
|
|
PER (%)## |
1.11ab |
1.15a |
1.03b |
0.75c |
0.51d |
<.001 |
|
|
Feed conversion ratio |
2.08c |
2.00c |
2.24c |
3.10b |
4.61a |
<.001 |
|
|
HSI (%)### |
1.28ab |
1.41a |
1.08ab |
1.06bc |
0.78c |
<0.001 |
|
|
Yield (kg/m3) |
9.58a |
9.36a |
8.09a |
4.06b |
1.17c |
0.001 |
|
| abcd: Means in the same row without common letter are different at p<0.05 #:Specific growth rate;##: Protein efficient ratio; ###: Hepatosomatic index | |||||||
 |
| Figure 2. The effect of Black soldier fly larvae on total weight gain |
In this study, the results showed that a replacement of up to 50% of fishmeal protein with BSFL protein (or 21.4% of DM diet) did not affect the productivity of the fish although the growth rate tended to decrease gradually. The results of this study are similar to the report of Katya et al (2018), who reported that inclusion of up to 30.8% BSFL in diets improved growth rate of seabass fingerlings with an initial weight of 6.7 g in a freshwater but not higher level of inclusion. Magalhăes et al (2017) indicated that inclusion of 19.5% BSFL didn’t affect growth performance of European seabass. However, Hoa and Dung (2016) found that inclusion of up to 30% BSFL as DM in diet improved daily weight gain of snake head fish. In study of Suong et al (2023) on gradually replacement of FM protein by BSFL (from 0% to 40%) in square-head climbing perch, the results showed that inclusion of BSFL increased slightly final weight (48.7 g vs 55.7-56.2 g), daily gain (0.72 g vs 0.85 g) and yield (1.2 kg/m2 vs 1.84 kg/m2) and improved FCR (1.75-1.82 vs 1.65-1.7). Similarly, Nghia et al (2023) reported that the replacement of up to 40% fishmeal protein by BSFL meal in diets for the Thai frogs improved the survival rate (75.6-86.8%) and FCR and increased final live weight (194-240.7 g) and daily weight gain (3.11-3.66 g) and frog’s yield (5.9-8.41 kg/m2). However, the report of Manh et al (2023) showed that replacing FM protein by BSFL at level of up to 30% did not affect the survival rate, growth rate and yield of snakehead fish but of 40% declined the growth performance as final weight, daily gain and fish yield. The study suggests that up to 12.9% BSFL meal can be included in diets without adverse effect on growth performance and yield of snakehead fish.
Previous studies have suggested that the reduction in growth performance of seabass with increasing larval meal content in the diet is due to the high chitin content and deficiency of essential amino acids such as cystine and lysine present in the larval meal (Katya et al 2018). In this study, increasing levels of BSFL in diets, the content of CF considering as mainly chitin content clearly increased (4.48% CF at AT0 as FM diet and 5.54-6.48% CF in AT50 and AT100).
Data in Table 3 showed that, the moisture content of fish fillet was higher in AT50-AT100 than in AT0 and AT25 (p<0.05); the contents of CP and EE in AT0 and AT25 were highest and lowest in AT100 (p<0.05). It means that replacement of FM protein by BSFL had significantly affect chemical composition of seabass fillet.
| Table 3. Chemical composition of seabass fillet (%) | |||||||
|
Item |
Treatment |
p -value |
|||||
|
AT0 |
AT25 |
AT50 |
AT75 |
AT100 |
|||
|
Moisture |
74.4b |
74.6b |
75.7a |
76.1a |
76.4a |
<.001 |
|
|
CP |
16.4a |
16.1ab |
15.7b |
15.2c |
14.3d |
<.001 |
|
|
EE |
3.36b |
4.94a |
2.92c |
2.47d |
1.67e |
<.001 |
|
|
Ash |
4.88b |
3.66c |
4.87b |
5.30a |
5.48a |
<.001 |
|
| abcde: Means in the same row without common letter are different at p<0.05 | |||||||
The findings in this study are similar to reports of Katya et al (2018), who found declining the contents of CP and EE in seabass raised in freshwater fed with FM replacement by larvae meal. Kroeckel et al (2012) found that increasing larval meal levels replacement of fishmeal in diets the EE content of turbot fillet declined. However, many studies have shown that the chemical composition of fish is not affected when replacing or supplementing different feed ingredients in the diet such as: when replacing 100% of fish meal with meat meal for olive flounder (Paralichthys olivaceus); in sea bream, replacing 70% of fish meal with poultry by-product meal; in sturgeon when replacing 100% of fish meal with larvae meal (Christian et al 2020). Further study on the effect of inclusion of larval meal in diets for fish may be needed.
Data on main digestible enzyme activities were presented in Table 4. In general, increasing BSFL protein levels of up to 50% as FM protein increased enzyme activity in both anteriorintestine (AI) and posterior intestine (PI), except for lipase.
|
Table 4. Digestible enzyme activity of seabass intestine |
|||||||
|
Item |
Treatment |
p -value |
|||||
|
AT0 |
AT25 |
AT50 |
AT75 |
AT100 |
|||
|
Protease (U/mg protein) |
|||||||
|
AI# |
0.40c |
0.79a |
0.55b |
0.39c |
0.30c |
<.001 |
|
|
PI## |
0.24d |
0.59b |
0.61b |
0.65a |
0.48c |
<.001 |
|
|
Pepsin (pmol/minute/mL) |
|||||||
|
AI |
1.08d |
1.38a |
1.37a |
1.25b |
1.17c |
<.001 |
|
|
PI |
1.07c |
1.38a |
1.37a |
1.24b |
1.15bc |
<.001 |
|
|
Stomach |
1.25ab |
1.26ab |
1.33a |
1.25ab |
1.14b |
0.011 |
|
|
Trypsin (nmol/minute/mL) |
|||||||
|
AI |
4.95d |
15.78a |
12.86b |
7.77c |
4.24d |
< .001 |
|
|
PI |
4.24c |
9.71b |
10.93b |
13.05a |
14.09a |
< .001 |
|
|
Lipase (U/mg protein) |
|||||||
|
AI |
5.81a |
5.25a |
4.36b |
5.23a |
5.83a |
0.001 |
|
|
PI |
1.59c |
2.91b |
3.40a |
3.11ab |
3.07ab |
<.001 |
|
|
Amylase (U/mg protein) |
|||||||
|
AI |
0.06a |
0.07a |
0.07a |
0.06a |
0.05a |
0.130 |
|
|
PI |
0.03d |
0.07bc |
0.09a |
0.08ab |
0.07c |
< .001 |
|
|
abcd: Means in the same row without common letter are different at p<0.05 #AI: Anterior intestine;##PI: Posterior intestine |
|||||||
Most fish species have enzymes to digest proteins, lipids and carbohydrates. However, the activity of these enzymes in the larval stage is often lower than that in adult fish. Some studies on the activity of digestive enzymes in fish show that enzyme activity is affected by diet and feeding habits. In addition, enzyme activity is also affected by intermittent or continuous feeding methods (Le Thi Tieu Mi et al 2013; Sangiao-Alvarellos et al 2005).
In this study, the protease value obtained in the AI was greater than the PI and increasing the replacement level of FM protein with BSFL protein to 50% reduced the protease enzyme activity. Digestive enzymes are necessary for the digestion of feed, especially proteins. Normally, to break down proteins into amino acids and smaller peptides before absorption in the small intestine, pepsin first partially hydrolyses proteins, followed by trypsin hydrolysis. The results of the study on catfish (Pseudoplatystoma corruscans) showed that most of the enzymes were found in the stomach and a small part in the foregut and no enzymes were found in the midgut and hindgut (Lundstedt et al 2004). Some studies have shown that rainbow trout and common carp have high protease activity, but carnivorous fish such as European eel (Anguilla anguilla) and sea bream have lower protease activity.
Similar to protease enzymes, lipase enzyme activity also varies depending on feeding habits. In herbivorous fish such as tilapia (Oreochromis niloticus), lipase activity is limited in the digestive tract (Tengjaroenkul et al 2000), while the omnivorous fish (Colossoma macropomum) shows high lipase activity in the stomach and intestine tract (Almeida et al 2006; Pérez-Jiménez et al 2009). Although lipase enzyme was not detected in the stomach of the carnivorous fish (Dentex dentex), it was detected throughout the digestive tract in some carnivorous species such as the catfish (Lundstedt et al 2004) and the Asian arowana (Scleropages formosus) (Natalia et al 2004) and the highest enzyme activity was observed in the intestine. In this study, seabass is a carnivorous fish that consumes high-fat diets and the occurrence of lipase enzyme in the digestive tract of fish is reasonable. Morever, the lipase enzyme activity in the AI is higher in the PI and is lower in the diet of 50% FM protein replaced by BSFL than in other treatments.
In this study, amylase enzyme in the AI ranged from 0.05 to 0.07 (U/mg protein) and there was no statistical difference between treatments. However, amylase enzyme activity in the PI showed high values in the AT50 and low values in the AT0. Normally, amylase enzyme is found in most fish species. Amylase enzyme activity depends a lot on feeding habits and is quite high in herbivorous and omnivorous fish than in carnivorous fish (Ji et al 2012). Therefore, the presence of amylase enzyme in fish species is different, high in omnivorous and herbivorous fish such as carp, tilapia and milkfish; low in marine carnivorous fish whose main food component is protein with very little carbohydrates such as sea bream (Sparus aurata), yellowtail (Serola quiquradiata), seabass and Dentex dentex (Eroldogan et al 2008; Pérez-Jiménez et al 2009). However, some carnivorous species such as rainbow trout and dolphin (Anarhichas minor) did not detect amylase enzyme in the digestive tract (Hidalgo et al 1999).
Normally, digestive enzymes such as protease, lipase and amylase are secreted in large quantities in the AI, so digestive enzyme activity will also be strong there. However, in this study, no clear difference in enzyme activity was shown in the digestive tract, similar to published results in European seabass (Magalhăes et al 2015). This could be explained by the fact that digestive enzyme activity and the transit time of digesta along the digestive tract can be affected by the proportion of feed ingredients in the diet (Castro et al 2013; Pérez-Jiménez et al 2009).
Results in Table 5 showed that URE, CRE, GLU, A/G, AST and TG were not different among treatments (p>0.05), but the value of TP was highest in AT50 and lowest in AT100 (p<0.05); meanwhile, the GLB, ALT and CHOL values in AT100 were lower than those in other dietary treatments (p<0.05).
|
Table 5. Haematological indices |
|||||||
|
Item# |
Treatment |
p -value |
|||||
|
AT0 |
AT25 |
AT50 |
AT75 |
AT100 |
|||
|
URE (mmol/L) |
1.94 |
2.16 |
2.31 |
2.02 |
2.06 |
0.075 |
|
|
CRE (µmol/L) |
10.1 |
11.3 |
10.2 |
11.7 |
8.78 |
0.184 |
|
|
GLU (mmol/L) |
6.94 |
7.27 |
6.99 |
6.40 |
4.51 |
0.141 |
|
|
TP (g/L) |
39.0ab |
39.4ab |
41.7a |
37.6bc |
35.8c |
0.003 |
|
|
ALB (g/L) |
12.6a |
12.7a |
13.3a |
12.7a |
11.5b |
0.001 |
|
|
GLB (g/L) |
26.4ab |
26.7ab |
28.4a |
25.9ab |
24.9b |
0.058 |
|
|
A/G |
0.48 |
0.48 |
0.47 |
0.45 |
0.44 |
0.331 |
|
|
AST (u/L) |
183.9 |
149.3 |
195.4 |
177.6 |
168.7 |
0.119 |
|
|
ALT (u/L) |
47.6b |
43.8b |
55.7b |
68.3a |
70.1a |
<.001 |
|
|
CHOL (mmol/L) |
4.43a |
4.52a |
4.89a |
4.38ab |
3.90b |
0.009 |
|
|
TG (mmol/L) |
2.41 |
2.29 |
2.70 |
2.36 |
2.11 |
0.121 |
|
|
abc: Means in the same row #URE: urea; CRE: creatinine; GLU: glucose; TP: total protein; ALB: albumin; GLB: globulin; A/G: albumin/globulin ratio; AST: aspartate aminotransferase; ALT: alanine transaminase; CHOL: cholesterol; TG: triglycerides |
|||||||
Haematological indices are widely used in assessing the nutritional status, health and adaptability of fish to the external environment (Abdel-Tawwab 2016; Burgos-Aceves et al 2019). Haematological indices of fish are an important index to monitor physiological and pathological changes that may arise when evaluating novel feeds (Madibana et al 2017; Ahmad et al 2021). Meanwhile, URE and CRE are the final products of nitrogen metabolism, providing information on kidney function assessment (Ajeniyi et al 2014). Blood glucose (GLU) levels change during stress in aquatic animals. Blood GLU increases to provide energy for animals to respond to stress. TP concentration in blood plays an important role, performing the functions of protection, regulation and transport of various substances in the organism (Sharaf and Khan, 2020). Serum AST and ALT values are associated with liver damage or liver necrosis when they increase. Cholesterol (CHOL) in blood plays an important role in the synthesis of cell membranes, bile and hormones in animals including fish (Tan et al 2016). Although fish have the ability to synthesize cholesterol and the majority of cholesterol in blood comes from the liver and from the digestive tract. In fish, the hypocholesterolaemia effect of plant proteins has been found, but very little information has been reported on animal proteins (Shafaeipour et al 2008).
Hender et al (2021) reported that total TP content in sea bass fingerlings was not affected when replacing FM protein with BSFL protein up to 30%. Similarly, it was not affected when replacing up to 20% in Argyrosomus japonicus (Madibana et al 2020); and also replacing up to 100% in common carp (Li et al 2017). Meanwhile, GLB index has also been shown to be able to regulate immune response in fish. On the other hand, TP concentration is related to ALB and GLB as increased concentration in immune disorders and liver dysfunction (Banaee et al 2011; John 2007). In this study, increasing levels of FM protein replacement by BSFL in the diet, the TP, ALB and GLB serum indices tended to decrease but were not different from the control diet, which indirectly showed that the ratio of larvae replacing fish meal in the diet may not affect the functions of the immune system in fish.
The results of Madibana et al (2020) on Argyrosomus japonicus showed that ALT content increased with increasing larval supplementation levels, but not in Atlantic salmon (Belghit et al 2018). In this study, AST content was stable but serum ALT tended to increase with increasing FM protein replacement with BSFL meal, with a replacement level of above 50%, the ALT content in BSFL diets had a statistically significant difference compared to the control diet. Therefore, seabass may be affected in liver function if FM protein is replaced with BSFL meal at a level higher than 50%, this needs further research to have accurate conclusions.
The hypocholesterolaemia effect in common carp was recorded when fed with feed supplemented with larvae meal compared to the control (Li et al 2017; Ji et al 2015). According to Lindsay (1984), the ability to reduce blood cholesterol is due to the high chitin content of BSFL (8.7% DM) that can be degraded by chitinase in the fish body. Chitosan is the main product of chitin hydrolysis by chitinase enzyme, which some studies have shown to reduce cholesterol levels in animal blood (Chen et al 2014). In this study, event high chitin content in BSFL meal but the chitin content in the diets was low (ranged 5.05-6.45% CF) compared to the recommendation of Lindsay (1984), so the effect of reducing blood cholesterol was unclear.
Partly and fully replacing fishmeal protein by BSFL in the diets were negative affected on growth performance and survival rate at levels of above 50% (or 21.4% as DM diet) but did not affect the chemical composition of meat and some digestive enzymes as well as haematological indicators of seabass fingerlings raised in freshwater. In recommendation, inclusion of up to 21.4% as DM full-fat BSFL meal can be included in the diets for seabass fingerlings.
This work was supported by Hue University under the Core Research Program, Grant No. NCTB.DHH.2025.14.
Abdel-Tawwab M 2016 Effect of feed availability on susceptibility of Nile tilapia, Oreochromis niloticus (L.) to environmental zinc toxicity: growth performance, biochemical response and zinc bioaccumulation, Aquaculture 464, 309–315.https://doi.org/10.1016/j.aquaculture.2016.07.009
Ajani F, Dawodu M O and Bello-Olusoji O A 2011 Effects of feed forms and feeding frequency on growth performance and nutrient utilization of Clarias gariepinus fingerlings. African Journal of Agricultural Research , 6(2): 318-322. http://dx.doi.org/10.5897/AJAR10.714
Almeida D L C, Lundstedt L M and Moraes G 2006 Digestive enzyme responses of tambaqui (Colossoma macropomum) fed on different levels of protein and lipid. Aquaculture Nutrition,12: 443–450 . https://doi.org/10.1111/j.1365-2095.2006.00446.x
AOAC 1990 Official Methods of Analysis. 15th ed. Association of Official Analytical Chemists, Arlington, VA, USA: AOAC International.
AOAC 1997 Official Method 994.12 Amino Acids in Feeds. https://www.scribd.com/document/609975831/Aoac-Official-Method-994-12-Amino-Acids-in-Feeds-1
Banaee M, Sureda A, Mirvaghefi A R and Rafei G R 2011 Effects of long-term silymarin oral supplementation on the blood biochemical profile of rainbow trout (Oncorhynchus mykiss). Fish Physiological Biochemistry. 37 (4), 885–896. https://doi.org/10.1007/s10695-011-9486-z
Belghit I, Liland N S, Gjesdal P, Biancarosa I, Menchetti E, Li Y, Waagbř R, Krogdahl Ĺ, Lock E J 2018 Black soldier fly larvae meal can replace fishmeal in diets of sea-water phase Atlantic salmon (Salmo salar). Aquaculture, 503, 609–619. https://doi.org/10.1016/j.aquaculture.2018.12.032
Burgos-Aceves M A, Lionetti L, Faggio C 2019 Multidisciplinary haematology as prognostic device in environmental and xenobiotic stress-induced response in fish. The Science of the total environment, 670, 1170–1183. https://doi.org/10.1016/j.scitotenv.2019.03.275
Castro C, Perez-Jimenez A, Coutinho F, Pousao-Ferreira P, Brandao T M, Oliva Teles A and Peres H 2013 Digestive enzymes of meagre (Argyrosomus regius) and white seabream (Diplodus sargus): Effects of dietary brewer's spent yeast supplementation. Aquaculture 416: 322-327 https://ui.adsabs.harvard.edu/link_gateway/2013Aquac.416..322C/doi:10.1016/j.aquaculture.2013.09.042
Christian C, Manuela R, Carola L, Alessio B, Marta G, Marco M, Sihem D, Achille S, Francesco G, Antonia CE, Marino P and Laura G 2020 First insights on black soldier fly (Hermetia illucens L.) larvae meal dietary administration in Siberian sturgeon (Acipenser baerii Brandt) juveniles. Aquaculture, 515: 734539.https://doi.org/10.1016/j.aquaculture.2019.734539.
Cupp-Enyard C 2008 Sigma's non-specific protease activity assay-casein as a substrate. Journal of Visualized Experiments, 2008 (19):e899 .https://doi.org/10.3791/899
Eroldoğan O T, Taşbozan O and Tabakoğlu S 2008 Effects of restricted feeding regimes on growth and feed utilization of juvenile gilthead sea bream, Sparus aurata. Journal of the World Aquaculture Society, 39(2): 267-274.http://dx.doi.org/10.1111/j.1749-7345.2008.00157.x
Hassan H U, Qadeer M A, Zubia M and Mohammad A M S 2021 Growth performance and survivability of the Asian seabass Lates calcarifer reared under hypersaline hyposaline and freshwater environments in a Closed aquaculture system. Research Square, 1-15; https://doi.org/10.21203/rs.3.rs-211072/v1
Hender A, Muhammad AB, Siddik J H and Ravi F 2021 Black soldier fly, Hermetia illucens as an alternative to fishmeal protein and fish oil: Impact on growth, immune response, mucosal barrier status and flesh quality of juvenile Barramundi, Lates calcarifer (Bloch, 1790). Biology 10, 505. 1-17. https://doi.org/10.3390/biology10060505.
Hidalgo M C, Urea E and Sanz A 1999 Comparative study of digestive enzymes in fish with different nutritional habits. Proteolytic and amylase activities, Aquaculture, 170: 267–283. https://doi.org/10.1016/S0044-8486(98)00413-X
Irmawati I, Umar M T, Ambo Ala Husain A, Citra Malina A, Nurdin K N and Alimuddin A 2020 Distribution and characteristics of Asian seabass (Lates calcarifer Bloch, 1790) in South Sulawesi. IOP Conference Series: Earth and Environmental Science vol 564 (IOP Publishing), p. 012011. http://10.1088/1755-1315/564/1/012011
ISO 2017ISO 12966-2:2017 Specifies methods of preparing the methyl esters of fatty acids. International Standard confirmed [90.93]. https://www.iso.org/standard/72142.html
Ji H, Zhang J L, Huang J Q, Cheng X F and Liu C 2015 Effect of replacement of dietary fish meal with silkworm pupae meal on growth performance, body composition, intestinal protease activity and health status in juvenile Jian carp (Cyprinus carpio var. Jian), Aquaculture Research, 46 (5), 1209–1221.http://dx.doi.org/10.1111/are.12276
Katya K, Borsra M Z S, Ganesan D, Kuppusamy G, Herriman M, Salter A and Ali S A 2018 Efficacy of insect larval meal to replace fish meal in juvenile barramundi. Lates calcarifer reared in freshwater. International Aquatic Research. 9: 303-312; https://doi.org/10.1007/s40071-017-0178-x
Kim H J, Seong-Mok Jeong, Jin-Ho Bae, Kang-Woong Kim and Sang-Woo Hur 2024 Evaluation of black soldier fly Hermetia illucensmeal as a fish meal replacement for growing Red seabream Pagrus major. Korean Journal of Fish Aquatic Science. 57(4):342-348,2024; http://doi.org/10.5657/kfas.2024.0342
Kroeckel S, Harjes A G E, Roth I, Katz H, Wuertz S, Susenbeth A and Schulz C 2012 When a turbot catches a fly: Evaluation of a pre-pupae meal of the black soldier fly (Hermetia illucens) as fish meal substitute-Growth performance and chitin degradation in juvenile turbot (Psetta maxima). Aquaculture, 364: 345-352. https://doi.org/10.1016/j.aquaculture.2012.08.041
Lan P T P, Ngoan L D, Quan N H and Tram N D Q 2022 Effects of harvesting time on yield, chemical composition of black soldier fly (Hermetia illucens) larvae and replacement of trash fish for feeding seabass (Lates calcarifer Bloch, 1790) rearing in fresh and brackish water. Livestock Research for Rural Development. Volume 34, Article #3. Retrieved from http://www.lrrd.org/lrrd34/1/3403ndqtr.html
Li S, Ji H, Zhang B, Zhou J and Yu H 2017 Defatted black soldier fly (Hermetia illucens) larvae meal in diets for juvenile Jian carp ( Cyprinus carpio var. Jian): Growth performance, antioxidant enzyme activities, digestive enzyme activities, intestine and hepatopancreas histological structure. Aquaculture 477, 62-70, http://dx.doi.org/10.1016/j.aquaculture.2017.04.015
Lindsay G J H, Walton M J, Adron J W, Fletcher T C, Cho C Y and Cowey C B 1984 The growth of rainbow trout(Salmo gairdneri)given diets containing chitin and its relationship to chitinolytic enzymes and chitin digestibility. Aquaculture 38, 315–334. DOI: 10.1016/0044-8486(84)90297-7
Lundstedt LM, Bibiano J F and Moraes G 2004 Digestive enzymes and metabolic profile of Pseudoplatystoma corruscans (Teleostei: Siluriformes) in response to diet composition, Comparative Biochemistry and Physiology, 137: 331– 339. https://doi.org/10.1016/j.cbpc.2003.12.003
Madibana M J, Mlambo V, Lewis B R and Fouche C H 2017 Effect of graded levels of dietary Ulva sp. on growth, hematological and serum biochemical parameters in dusky kob, (Argyrosomus japonicus, Sciaenidae). The Egyptian Journal of Aquatic Research43, 249–254. http://dx.doi.org/10.1016/j.ejar.2017.09.003
Madibana M J, Mwanza M, Lewis B R, Fouché C H, Toefy R and Mlambo V 2020 Black soldier fly larvae meal as a fishmeal substitute in juvenile Dusky Kob diets: Effect on feed utilization, growth performance and blood parameters. Sustainability 12, 9460,1-11. https://doi.org/10.3390/su12229460
Magalhăes R, Sánchez-López A, Leal R S, Martínez-Llorens S, Oliva-Teles A and Peres H 2017 Black soldier fly (Hermetia illucens) pre-pupae meal as a fish meal replacement in diets for European seabass (Dicentrarchus labrax). Aquaculture, 476: 79-85. https://doi.org/10.1016/j.aquaculture.2017.04.021
Manh H N, Lan P T P, Tue L M, Thuy N T T, Hoa T T, Nghia V D, Suong T T T and Tram N D Q 2023 Response on growth performance and chemical composition of fillet of snakehead fish (Channa sp.) fed diets composed black soldier fly larvae protein replacing fishmeal protein. Livestock Research for Rural Development. Volume 35, Article #88. Retrieved August 29, 2024, from http://www.lrrd.org/lrrd35/10/3588ndqu.html
Mathai E N, Barwani D K, Mwashi V, Munguti J M, Iteba J, Wekesa F, Tanga C M, Kiiru P, Gicheha M G and Osuga I M 2024 Black soldier fly (Hermetia illucens) larvae for Nile tilapia (Oreochromis niloticus) on-farm. Journal of Agriculture Science and Technology. 23(3):120-143. https://doi.org/10.4314/jagst.v24i3.8
Miller G L 1959 Use of dinitrosalicylic acid reagent for determination of reducing sugar. Analytical Chemistry.
Minitab 2010 Minitab Inc. US. Licensing 16.2.0.0
Nghia V D, Lan P T P and Tram N D Q 2023 Effect of replacement of fishmeal by black soldier fly larvae meal in diets on growth performance, carcass traits and meat chemical composition of Thai frog ( Rana rugosa Temminck and Schelegel, 1838). Livestock Research for Rural Development. Volume 35, Article #76. Retrieved August 29, 2024, from http://www.lrrd.org/lrrd35/8/3576ndqt.html
Nhan D T, Tu N P C and Tu N V 2022 Comparison of growth performance, survival rate and economic efficiency of Asian seabass (Lates calcarifer) intensively cultured in earthen ponds with high densities. Aquaculture, 554: 738151. https://doi.org/10.1016/j.aquaculture.2022.738151
Pérez-Jiménez A, Cardenete G, Morales A, Garcia-Alcazar A, Abellan E and Hidalgo M 2009 Digestive enzymatic profile of Dentex dentex and response to different dietary formulations. Comparative Biochemistry and Physiology Part A: Molecular & Integrative Physiology154, 157- 164. https://doi.org/10.1016/j.cbpa.2009.05.126
Pethiyagoda R and Gill A C 2012 Description of two new species of sea bass (Teleostei: Latidae: Lates) from Myanmar and Sri Lanka. Zootaxa 3314: 1-16. https://doi.org/10.11646/zootaxa.3314.1.1
Sangiao-Alvarellos S, Guzmán J M, LáizCarrión R, Míguez J M, Marín Del Río M P, Mancera J M and Soengas J L 2005 Interactive effects of highstocking density and food deprivation on carbohydrate metabolism in several tissues of gilthead sea bream Sparus aurata. Journal of Experimental Biology 303(A): 761-775. https://doi.org/10.1002/jez.a.203
Schipp G, Bosmans J and Humphrey J 2007 Barramundi Farming Handbook. 3rd Ed., 23–24pp. Dept of Primary Industry, Fisheries and Mines, Northern Territory Govn., Australia. https://www.researchgate.net/publication/268349451_Barramundi_Farming_Handbook
Shafaeipour A, Yavari V, Falahatkar B, Maremmazi J G and Gorjipour E 2008 Effects of canola meal on physiological and biochemical parameters in rainbow trout (Oncorhynchus mykiss). Aquaculture Nutrition. 14 (2), 110–119. https://doi.org/10.1111/j.1365-2095.2007.00509.x
Sharaf Y and Khan M A 2020 Effect of dietary isoleucine level on growth, protein retention efficiency, haematological parameter, lysozyme activity and plasma antioxidant status of fingerling Channa punctatus (Bloch). Aquaculture Nutrition, 00, 1–13. https://doi.org/10.1111/anu.13049
Suong T T T, Lan P T P, Tue L M, Thuy N T T, Hoa T T, Manh H N and Tram N D Q 2023 Effect of partly replacement of fishmeal protein by black soldier fly larvae protein on growth performance and meat chemical composition of square-head climbing perch ( Anabas testudineus Bloch, 1792). Livestock Research for Rural Development. Volume 35, Article #82. Retrieved August 29, 2024, from http://www.lrrd.org/lrrd35/9/3582ndqt.html
Tan X, Lin H, Huang Z, Zhou C, Wang A, Qi C and Zhao S 2016 Effects of dietary leucine on growth performance, feed utilization, non-specific immune responses and gut morphology of juvenile golden pompano Trachinotus ovatus. Aquaculture, 465:100–107. https://doi.org/10.1016/j.aquaculture.2016.08.034.
Tengjaroenkul B, Smith B J, Caceci T and Smith S A 2000Distribution of intestinal enzyme activities along the intestinal tract of cultured tilapia, Oreochromis niloticus L. Aquaculture, 182: 317–327.https://doi.org/10.1016/S0044-8486(99)00270-7
Vij S, Purusthaman K, Gopikrssna G, Bashee V S, Gopalakrishnan A, Hossain M S, Sivasubbu S, Scaria V, Jena J K, Ponniah A G and Orban L 2014 Barcoding of Asian seabass across its geographic range provides evidence for its bifurcation into two distinct species. Frontiers in Marine Science 1 (30):1-17. https://doi.org/10.3389/fmars.2014.00030